Abstract
PURPOSE: Diversity, equity, and inclusion (DEI) are core principles in medical education and essential elements in eliminating health and workforce disparities. Over the past several years, institutional DEI initiatives have led to increases in the numbers of underrepresented trainees and faculty at Yale School of Medicine (YSM) and Yale Cancer Center (YCC). In 2021, the Yale Hematology/Oncology (HO) Fellowship Program created a DEI curriculum for Yale HO Fellows, designed by the HO Chief Fellows with guidance from Fellowship Program Leadership and the Vice Chair of DEI for YCC. The goals of the DEI curriculum were to foster a programmatic culture of inclusion and empower fellows to identify and respond to discrimination events in the workplace.
METHODS: A baseline needs-based assessment was performed with online anonymized surveys. The six-question survey was designed to assess topic preferences and witnessed discrimination events; all HO fellows (n = 27) were emailed a link to the survey. Based on the needs assessment, a five part DEI curriculum was designed, which included 1) an interactive session with the Vice Chair of DEI, 2) a journal club with the Deputy Dean and Chief Diversity Officer at YSM, 3) an interactive session on advancing gender equity through allyship and awareness led by external consultants, 4) an interactive session on gender identity with two experts on LGBTQIA2S+ issues in medicine following a YCC Grand Rounds seminar, and 5) a Chief Fellow-led interactive session on upstander training. The DEI curriculum was mandatory for all fellows. Evaluations of the curriculum at its completion were collected via a separate online anonymous survey that included 11 questions about comfort level in addressing discrimination and harassment (Kirkpatrick level 2). Perceptions of DEI among HO fellows and Core Faculty were further gauged using annual trainee and faculty surveys distributed by the American Council of Graduate Medical Education (ACGME) in 2021 and 2022, and a separate survey designed and distributed by the Program Leadership to gauge perceptions of DEI among HO Core Faculty in 2021 and 2022.
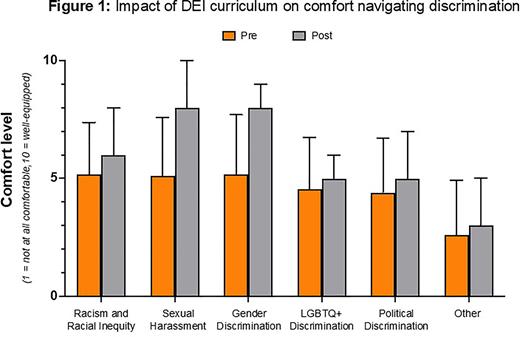
RESULTS: In total, 17 fellows (68%) responded to the pre-curriculum needs assessment survey. The majority of respondents witnessed (>75%) or experienced (>64%) some form of harassment during medical training. All respondents reported being under-equipped to navigate these scenarios. Inclusion of DEI into the fellowship curriculum was perceived as very important (mean 8.19/10, range 2-10). All fellows completed the five-part DEI curriculum. A total of 8 fellows (32%) responded to the post-curriculum survey; respondents reported an increase in comfort level navigating discrimination events (Figure 1). In ACGME trainee and faculty surveys prior to (2021) and following (2022) initiation of the DEI curriculum, compliance with DEI efforts increased above national means after initiation of the DEI curriculum (2021 mean scores, HO fellowship program vs national: faculty, 4.6 vs 4.4, fellows 4.2 vs 4.3; 2022: faculty 4.8 vs 4.5, fellows 4.6 vs 4.3). In surveys prior to and following the institution of the DEI, the percentages of HO Core Faculty who agreed that the HO Fellowship Program treats DEI as being core to its mission, on par with clinical excellence and investigative pursuits, increased from 71.4% to 80%. Those who agreed that DEI topics are formally or informally discussed as part of the educational experience of HO fellows increased from 28.5% to 50%.
CONCLUSION: A DEI curriculum for HO fellows may lead to significant improvements in fellow upstander responses to discrimination in the workplace and may augment institutional perceptions of DEI among fellows and faculty. We plan to continue our fellow-led DEI curriculum in perpetuity as part of our fellowship program and institution's commitment to DEI efforts.
Disclosures
Podoltsev:Blueprint Medicines: Honoraria; Agios Pharmaceuticals: Honoraria; Celgene: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Cogent Biosciences: Other: Independent Data Review Committee ; CTI BioPharma: Honoraria; PharmaEssentia: Honoraria; AbbVie: Honoraria; Pfizer: Honoraria; Bristol-Myers Squibb: Honoraria; Constellation Pharmaceuticals: Honoraria. Goldberg:AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Amgen, Blueprint Medicine, Sanofi Genzyme, Daiichi-Sankyo, Regeneron, Takeda, Janssen, and MIrati: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Burtness:Cue Biopharma: Research Funding; Merck: Research Funding; Gilead: Research Funding; Exelexis: Research Funding; Vaccinex: Research Funding; Genentech, Glaxo Smith Kline, Nektar, Debio, Merck KgA, Astra Zeneca, Vaccinex, Exelexis, Cue BioPharma, Fusion, Arvinas, Coherus, ALX Oncology, Hookipa: Consultancy. Isufi:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; BEAM Therapeutics: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Kite: Speakers Bureau. Kunz:RayzeBio: Membership on an entity's Board of Directors or advisory committees; Novartis (Advances Accelerator Applications: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen, Genentech, Crinetics, Natera, HutchMed, Ipsen, ITM: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal